How Can the Shape of a Molecule Determine Its Polarity
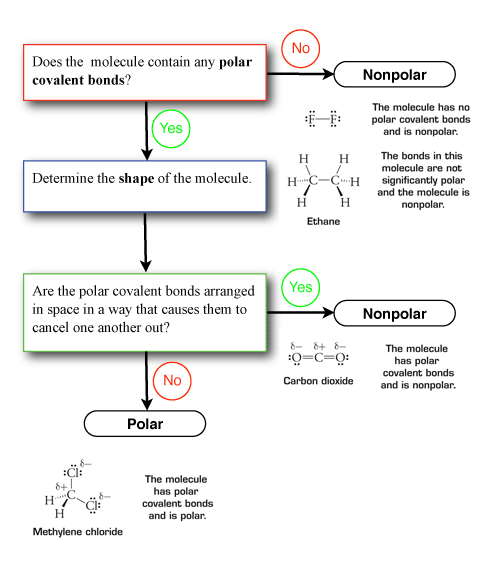
A tetrahedral molecule will never be a polar molecule. A symmetrical molecule will only have nonpolar bonds.
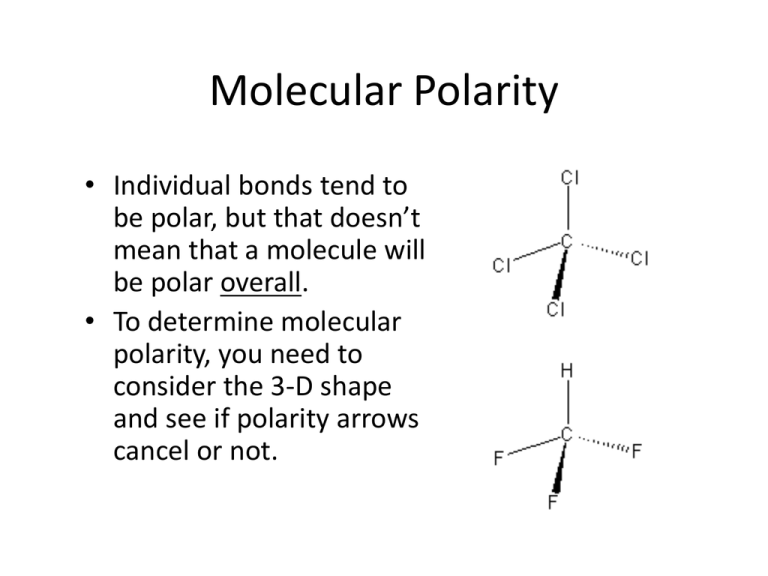
The shape of the molecule will determine the direction of each of the individual bond dipoles and thus will always play a role in determining the polarity of the molecule as a whole.
. Asymmetrical molecule cancels out the effects of polar bonds. 1 Show answers Another question on Chemistry. Add all of the bonds.
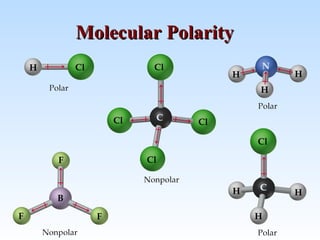
A polar molecule is one in which one side or end of the molecule has a slight positive charge and the other side or end has a slight negative charge. How can the shape of a molecule determine its polarity. A symmetrical molecule will only have nonpolar bonds.
However when there are more than two atoms involved in making a bond there are many complexities. This tells us that the bond dipole is a vector quantity. Draw the Lewis Structure The Lewis dot structure provides a simple model between the bonds in a molecule and the lone electron pairs.
A symmetrical molecule cancels out the effects of polar bonds. The shape of the molecule will determine the direction of each of the individual bond dipoles and thus will always play a role in determining the polarity of the molecule as a whole. Thus we can say that the shape of a molecule affect the polarity of the molecule.
Name the element in group 17 period 2. As learned before non-polar molecules are perfectly symmetrical while polar molecules are not. Choice A symmetrical molecule cancels out the effects of polar bonds.
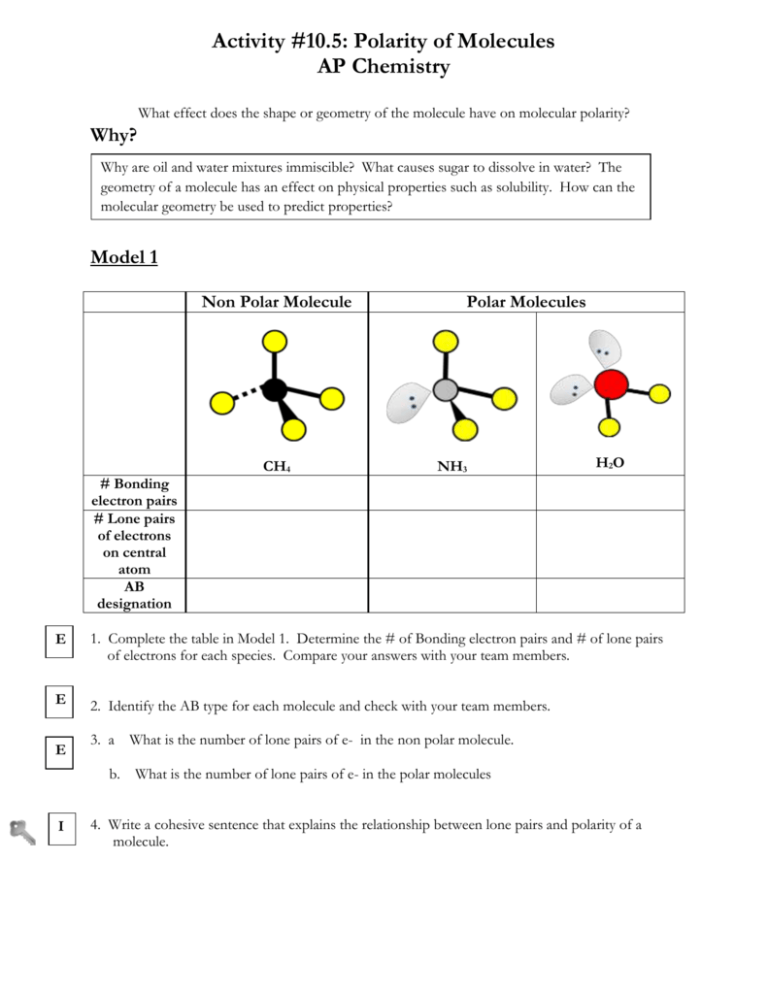
Begin drawing the Lewis dot structure of the molecule. Can the shape of a molecule tell you about it polarity. The polarization of a molecule greatly depends on the shape of the molecule.
Think of each polar bond in a molecule as a little arrow pointing from positive to negative. Once we know its shape we can determine whether a molecule is polar or nonpolar. This tells us that the bond dipole is a vector quantity.
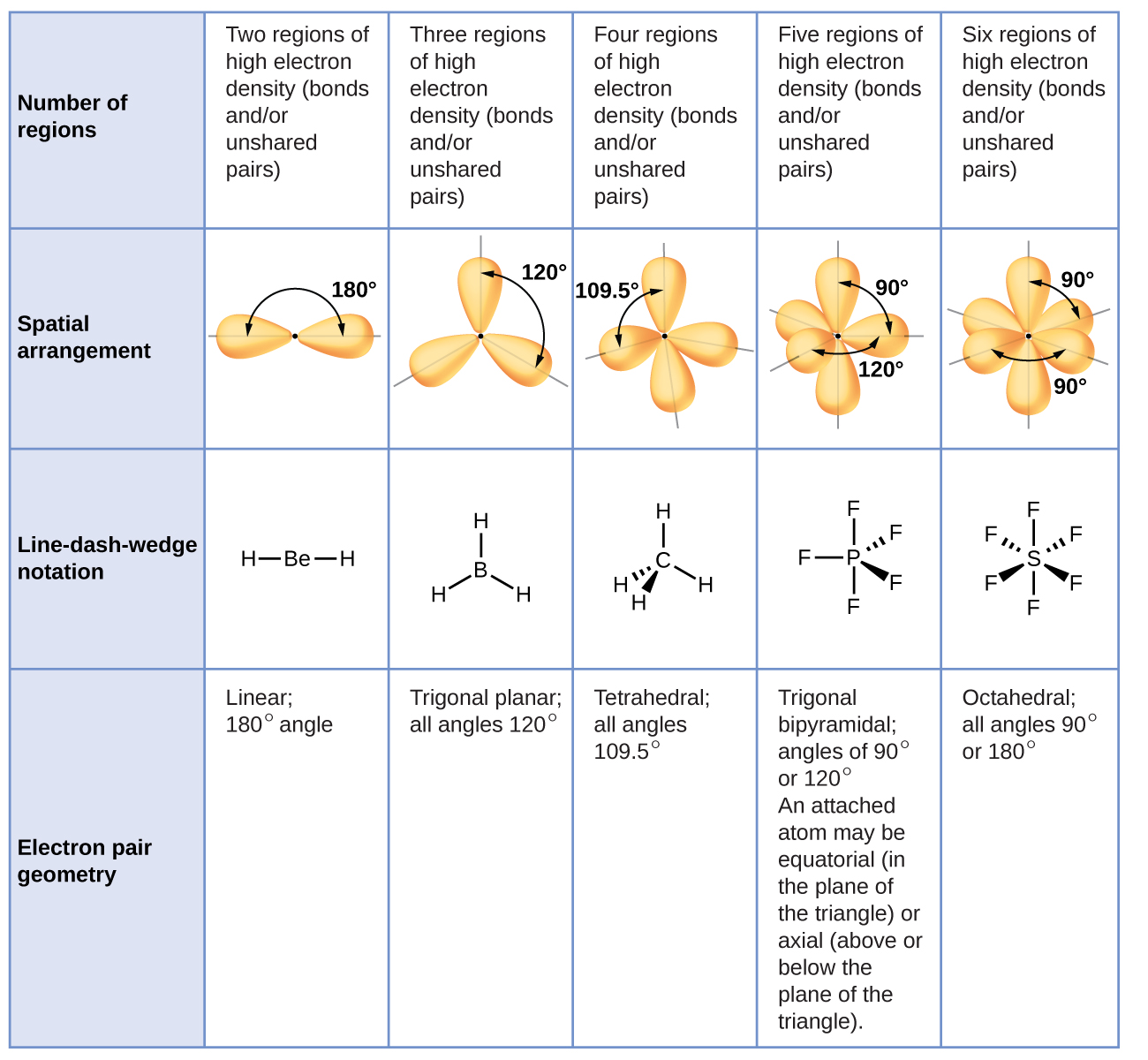
This means that if the shape of the molecule given to you is a bent or trigonal pyramid it is a polar molecule. The approximate shape of a molecule can be predicted from the number of electron groups and the number of surrounding atoms. How can the shape of a molecule determine its polarity.
This tells us that the bond dipole is a vector quantity. Its essential for predicting molecular geometry molecule polarity and reactivity in a compound. The overall polarity of molecules with more than one bond is determined from both the polarity of the individual bonds and the shape of the molecule.
A linear molecule will always contain polar bonds. If a molecule forms a symmetric shape the dipole moments on each molecule cancel out making the molecule nonpolar. Like any other vector quantity the.
The approximate shape of a molecule can be predicted from the number of electron groups and the number of surrounding atoms. Remember that asymmetry applies even if the outer atoms are the same. Exercises Questions What is the basic premise behind VSEPR.
In a water molecule add a single bond from the oxygen to both hydrogens. Think of each polar bond in a molecule as a little arrow pointing from positive to negative. The arrangement of the atoms matters more.
Some atoms may be double or triple bonded to achieve this. The shape of a molecule is another aspect that can determine its polarity. How can the shape of a molecule determine its polarity.
The molecular polarity can be established by determining the vector sum of all bond dipoles. A tetrahedral molecule will never be a polar molecule. So the geometry of the molecule determines the direction that the bond dipole vectors point.
The answer is 323 g. Use the octet rule to determine the number and type of bonds present. The molecular polarity can be established by determining the vector sum of all bond dipoles.
Each bonds dipole moment can be treated as a vector quantity having a magnitude and direction. 3 Steps to Determine if a Molecule is Polar Or Nonpolar 1. A linear molecule will always contain polar bonds.
Asymmetrical molecule will only have nonpolar bonds. To determine whether a molecule is polar. Each atoms valence shell should contain 8 electrons for the molecule to be stable.
If the molecule has a linear shape then it is not polar most of the time. How can the shape of a molecule determine its polarity. Particle model to predict what will happen if a sharp object creates a hole in the soccer ball.
If the molecule has different shaped elements in. A linear molecule will always contain polar bonds. Like any other vector quantity the.
A diatomic molecule like HF mentioned above has no issue of shape. This video looks at how to determine polarity in a molecule by understanding how the bond polarities molecule shape and outside atoms influence polarity us. The polarity of the.
The shape of the molecule will determine the direction of each of the individual bond dipoles and thus will always play a role in determining the polarity of the molecule as a whole. This will occur whenever the molecule is not completely symmetric. A tetrahedral molecule will never be a polar molecule.
Like any other vector quantity the direction is a vital aspect of its description. Think of each polar bond in a molecule as a little arrow pointing from positive to negative. The overall center of overlapping positive and negative charges determines whether a complex molecule is a nonpolar compound.
The net dipole moment is only due to the uneven distribution of electrons between the two atoms.

Polarity In Vsepr Shapes Youtube
How To Predict Polarity Of Molecules Based On Their Shape Biochemhelp
How To Tell If A Molecule Is Polar Or Non Polar Vsepr

Molecular Shape And Polarity How To Determine Whether A Molecule Will Be Polar Or Nonpolar Youtube
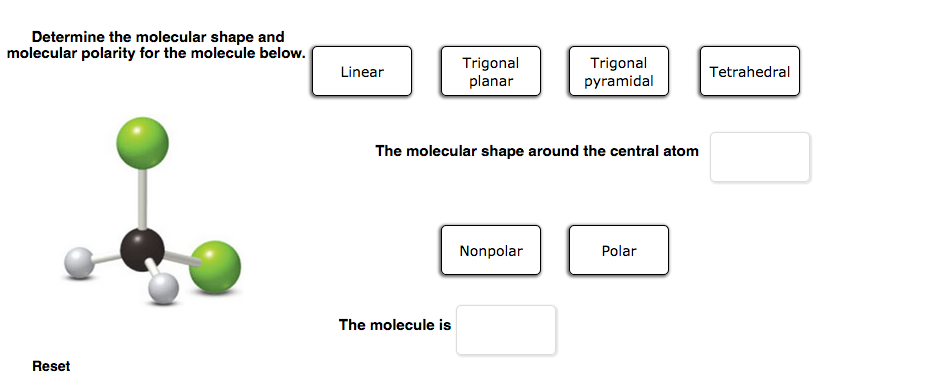
Solved Determine The Molecular Shape And Molecular Polarity Chegg Com

Polarity Of A Molecule Brilliant Math Science Wiki
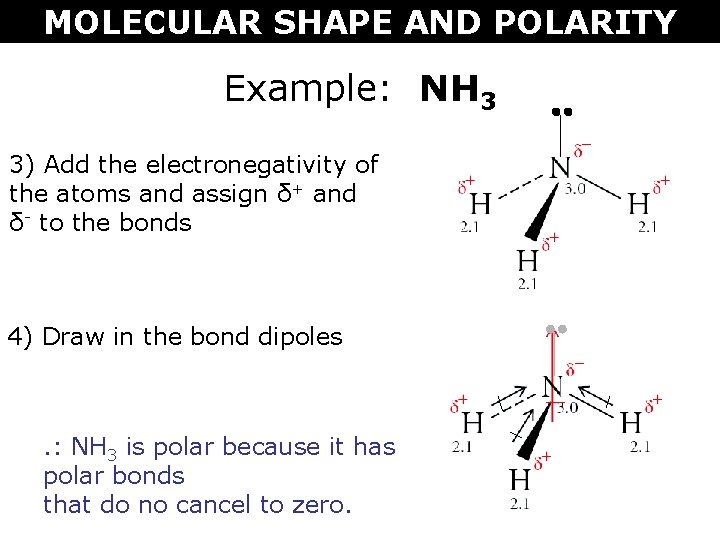
Molecular Shape And Polarity Molecular Shape And Polarity
Are Tetrahedral And Linear Shaped Molecules Always Non Polar Quora

Molecule Polarity Chemistry Tutorial Youtube
How Do Shape And Symmetry Influence The Polarity Of A Molecule Quora
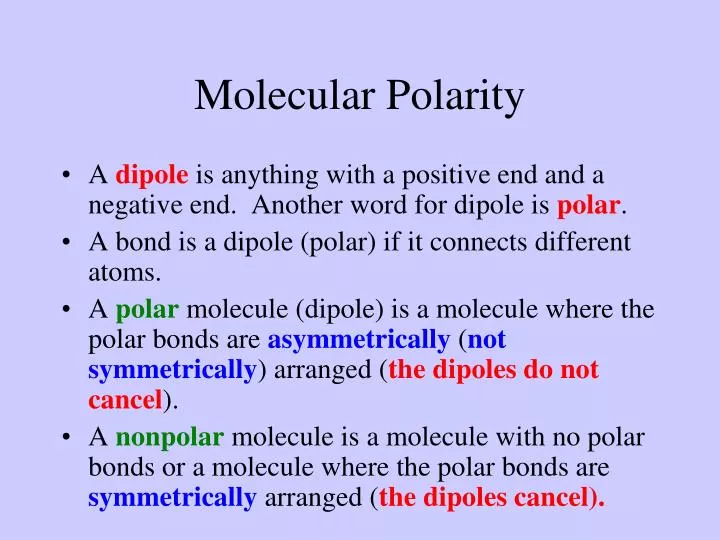
Ppt Molecular Polarity Powerpoint Presentation Free Download Id 2628490
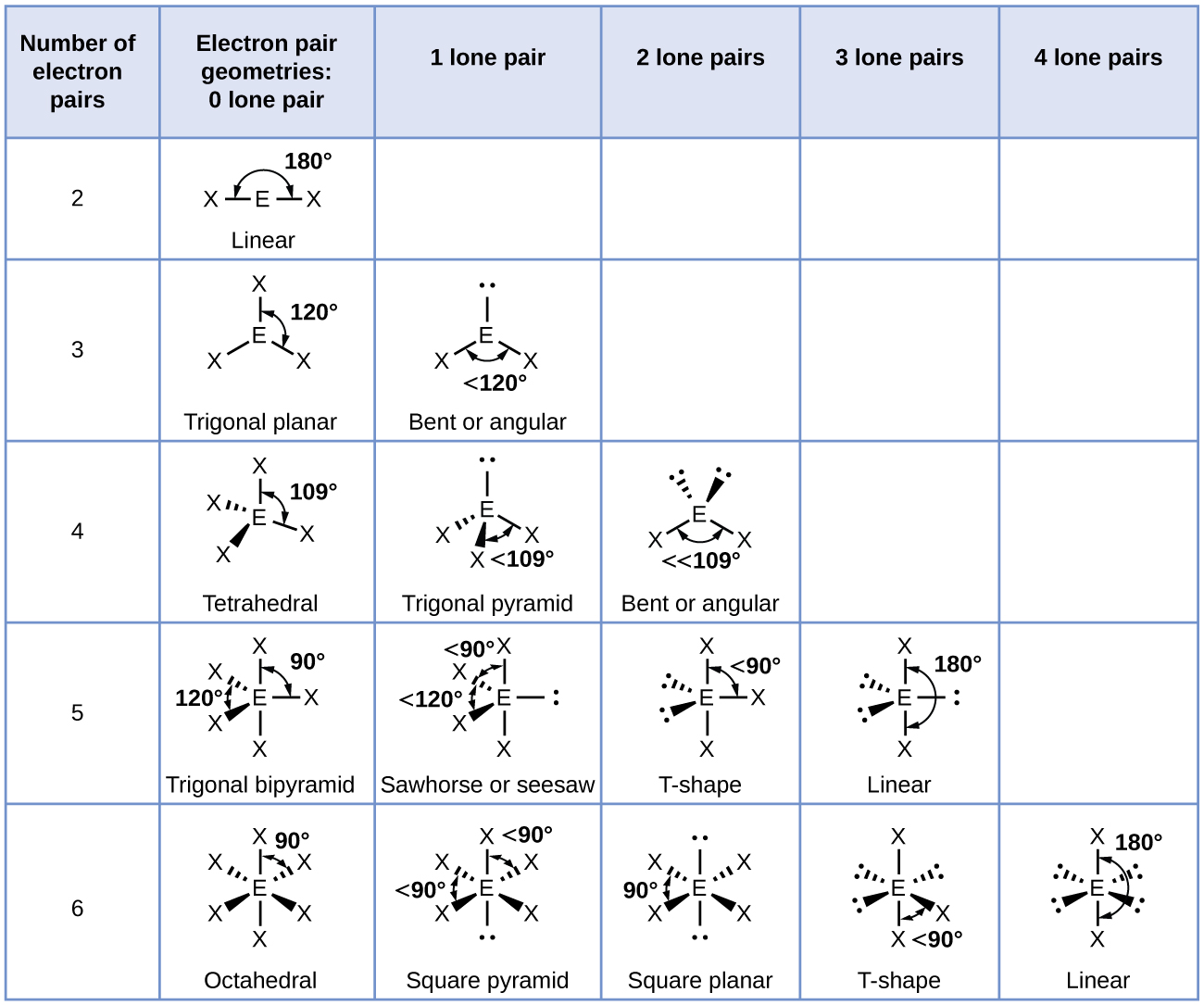
7 6 Molecular Structure And Polarity Chemistry
How Do Shape And Symmetry Influence The Polarity Of A Molecule Quora
How To Predict Polarity Of Molecules Based On Their Shape Biochemhelp
Unit 1 Elaboration Molecular Polarity

Comments
Post a Comment